Abstract
Introduction
At present, there is no standard of care for patients with relapsed/refractory non-Hodgkin lymphomas (NHL) after autologous stem cell transplantation (ASCT). Though, rituximab maintenance and interferon maintenance have been reported in patients with relapsed/refractory NHL after ASCT. Thalidomide is a kind of immunomodulatory drugs (IMiD) and has been proved to be effective in various NHLs, but the value of IMiD maintenance after ASCT remains to be discussed. In this study, we retrospectively analyzed the efficacy of thalidomide maintenance(TM) in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) after ASCT, comparing with historical control of patients without TM.
Patients and Methods
Between January 2013 and December 2017, eighty-eight patients with relapsed/refractory DLBCL undergoing TM after ASCT at the Sun Yat-sen University Cancer Center (SYSUCC) were reviewed. As historical control, ninety-seven patients without TM after ASCT during January 2006 to December 2012 were compared for analyses. All patients achieved CR before ASCT. Patients with transplantation related deaths were excluded. TM were given 100mg daily for one year. If grade III toxicities were observed, thalidomide would be postponed for one week and reduced to 75mg daily, and further 50mg daily in case of recurrence of grade III toxicities. The latest follow-up was updated to June 30th, 2018. Clinical and laboratory information was collected and analyzed.
Results
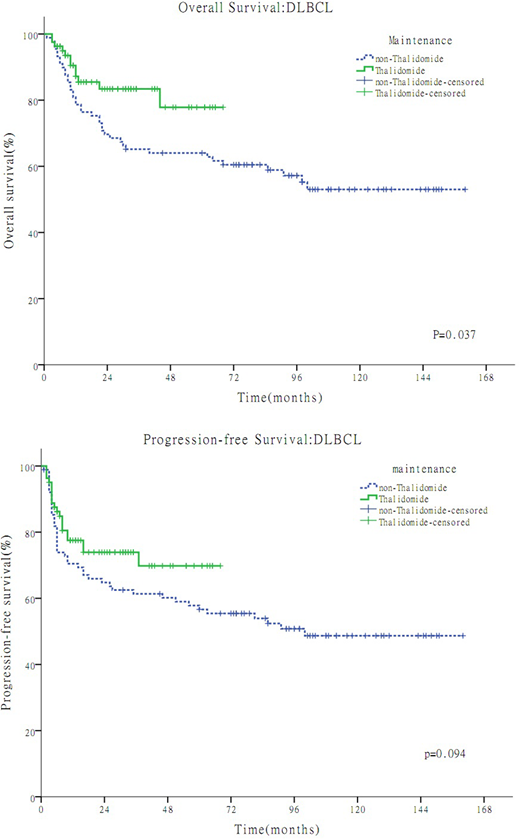
Overall, a total of 185 patients were reviewed and 15 of them were excluded due to lost to follow-up. In the TM group(n=81) and the non-TM group(n=89) available for survival analyses, the median age were 44 years (range, 19-72) and 40 years (range, 17-76) respectively, the proportion of male were 55% and 62% respectively, and the median follow-up time were 24 months(range, 6-68 months) and 79 months(range, 5-160 months) respectively. The 3-year overall survival (OS) were 77.8% in the TM group and 64.0% in the non-TM group, respectively (P =0.037). The 3-year progression-free survival (PFS) were 69.8% in the TM group and 61.4% in the non-TM group, respectively. (P=0.094). In multivariate analysis, maintenance therapy was an independent prognostic factor for OS (P < 0.05) .
The major toxicities observed in the TM group were as follows: thrombocytopenia(73.2%), leucopenia(50.0%), constipation(53.5%), fatigue(58.5%), Somnolence (38.7%), rash(25.0%), edema(23.0%), and peripheral neuropathy(8.3%).
Conclusions
Despite the retrospective nature and other limitations of this study, it is suggested that TM might be associates with promising survival benefit of in patients with relapsed/refractory DLBCL after ASCT, comparing with historical controls. It warrants further prospective studies to confirm the efficacy of IMiD maintenance in DLBCL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal